We are developing ZEN-3694 for the treatment of NUT carcinoma, a highly aggressive, rare type of cancer with very poor prognosis and no effective or approved therapies. ZEN-3694 received Fast Track designation from the FDA for its NUT carcinoma program
About NUT Carcinoma
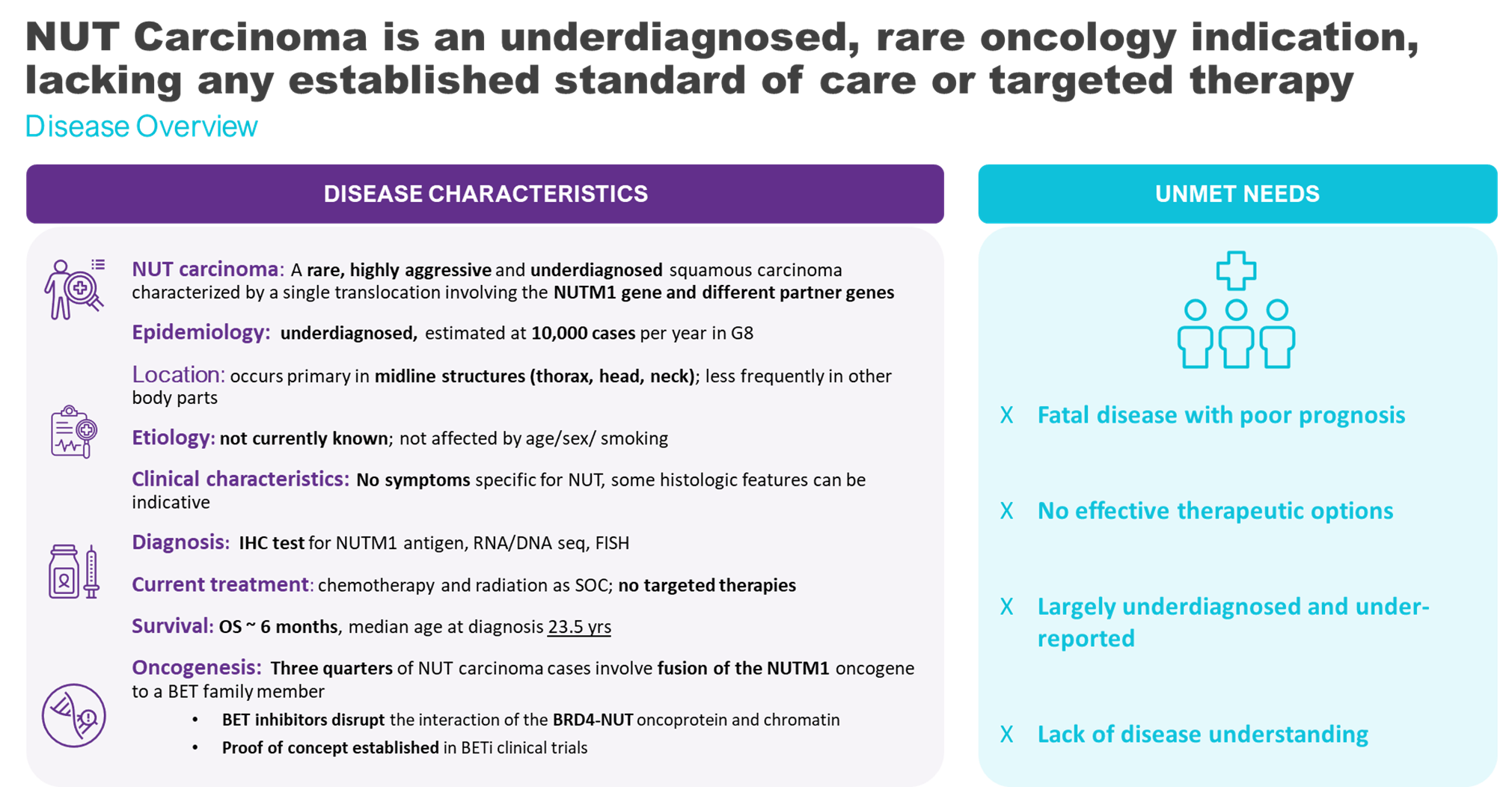
NUT carcinoma is a highly aggressive type of cancer affecting adults and children. It is a poorly differentiated squamous cell carcinoma that typically occurs in midline area of the body including head, neck, and thoracic areas. NUT carcinoma is characterized by the fusion of the NUTM1 gene to another gene to form an oncogene – a gene with the potential to cause cancer. Common fusion partners are the Bromodomain and Extra-terminal Domain (BET) proteins, BRD4 and BRD3, and NSD3. The NUT fusion onco-protein hijacks normal BET protein function, altering gene expression, and leading to unchecked tumor growth. ZEN-3694 is a BET inhibitor that binds to NUT fusion proteins, disrupts their activity, and inhibits cancer growth.
NUT Carcinoma Incidence
NUT carcinoma is a rare cancer, although it is also considered to be underdiagnosed - due to lack of awareness, misclassification, and suboptimal testing methods. The actual incidence of NUT carcinoma is estimated to be 10,000 cases per year in G8 countries. This is based on the incidence rate of NUT fusions in a large panel of sequenced tumors. The diagnosis rate is increasing rapidly with growing awareness and improved testing methods for this disease. Currently, the WHO classification of NUT carcinoma is being modified to squamous lung carcinoma and squamous Head and Neck Cancer. This along with the use of a straightforward immunohistochemistry rest (IHC) is expected to significantly increase the testing and diagnosis rates for lung carcinoma.
NUT Carcinoma Diagnosis
NUT carcinoma is diagnosed by detecting the NUTM1 fusion protein in a tumor biopsy through immunohistochemistry (IHC). The NUT fusion partner can also be identified by IHC, or by fluorescence in situ hybridization, DNA or RNA sequencing. These techniques, as well as growing awareness among clinicians, has increased the number of NUT carcinoma diagnoses, and enabled patient identification for the development of targeted therapeutics.
NUT Carcinoma Prognosis
An extremely aggressive cancer, NUT carcinoma is often resistant to treatment. Most patients are not diagnosed until the disease has become advanced locally or spread (metastasized) to other areas of the body. Despite aggressive treatment strategies, the median overall survival is approximately 6 months.
NUT Carcinoma Treatment
Surgery, radiation, and chemotherapy are used to treat NUT carcinoma, but chemotherapy is generally ineffective. There is no FDA approved therapy for this rare disease.
BET Inhibitors and NUT Carcinoma
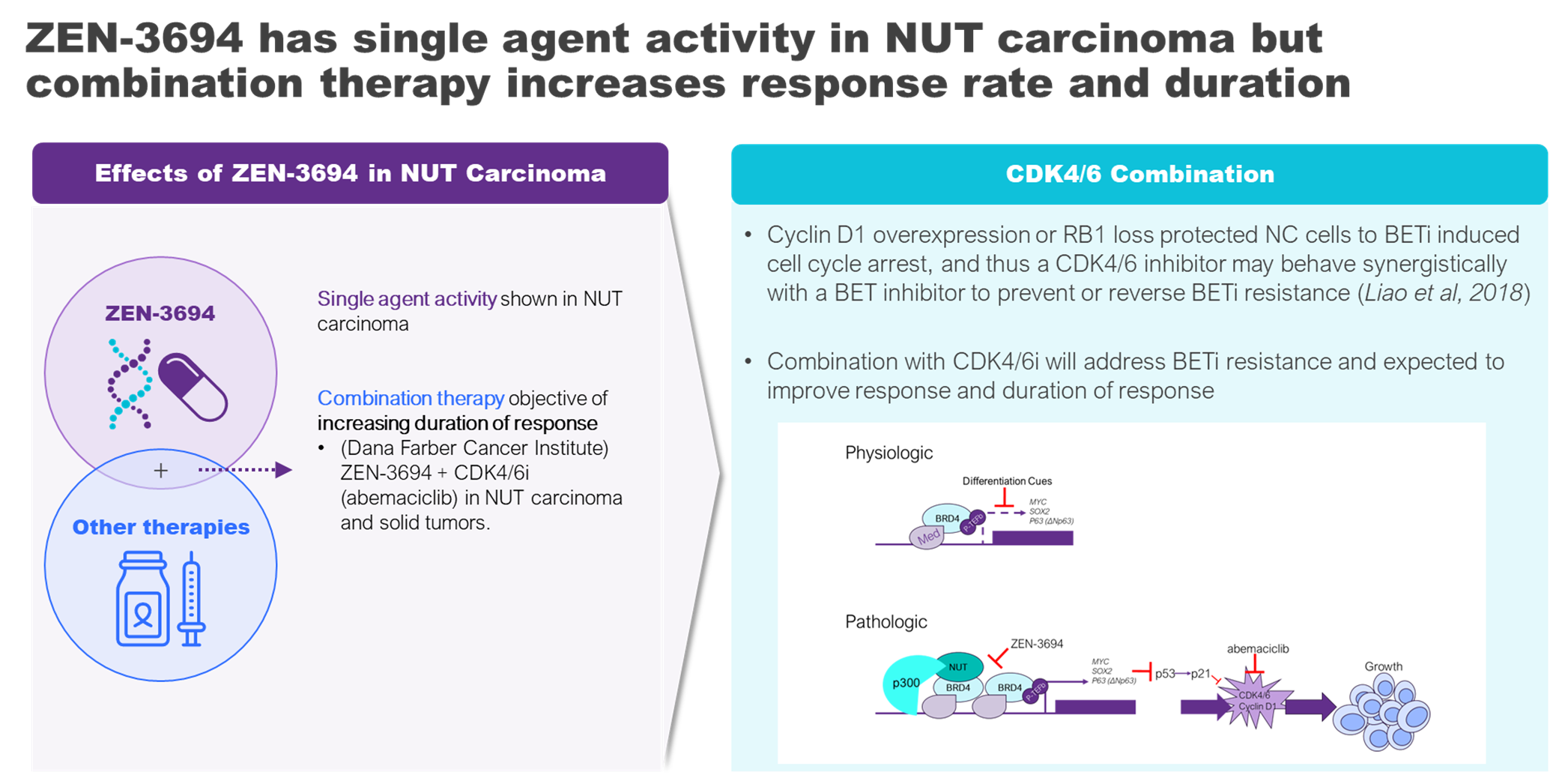
BET inhibitors have been shown to disrupt the function of the NUTM1 oncogenic fusion protein, the major driver of NUT carcinoma. While BET inhibitors have demonstrate some limited success in clinical evaluation, there is an unmet need for rational combination therapies which would improve the duration of response to BET inhibitors in this rare disease.
Zenith’s BET inhibitor, ZEN-3694, is being evaluated in two separate National Cancer Institute (NCI) sponsored clinical trials.
- A Phase 1 Study (NCT05372640) of ZEN-3694 in combination with CDK4/6 inhibitor abemaciclib in adult and pediatric patients (≥ 12 years old) with NUT carcinoma and other solid tumors.
- A Phase 1/2 Study (NCT05019716) of ZEN-3694 in combination with chemotherapy (cis-etoposide and platinum) in adult and pediatric NC patients (≥ 12 years old).
In addition to these clinical trials, Zenith has provided ZEN-3694 for compassionate use to patients with NUT carcinoma who have benefited from the treatment.
For further information on NUT carcinoma please visit The International NUT Carcinoma Registry or The NUT Carcinoma Alliance.