About Metastatic Castration Resistant Prostate Cancer
Prostate cancer is the fifth most common cause of male cancer death worldwide. Prostate cancer progression depends on androgens, such as testosterone. Tumors are often initially sensitive to medical or surgical therapies that decrease levels of testosterone and to therapies that block signaling from the androgen receptor (AR). However, disease progression may eventually lead to metastatic castration resistant prostate cancer (mCRPC) where the cancer has spread beyond the prostate to other parts of the body (metastatic) and continues to grow even when treatments to lower testosterone such as androgen deprivation therapy or surgical castration are no longer effective.
Metastatic castration-resistant prostate cancer (mCRPC) represents a significant and increasing challenge in oncology as patients develop resistance to existing treatments such as second-generation AR pathway inhibitors (ARPI).
mCRPC Incidence
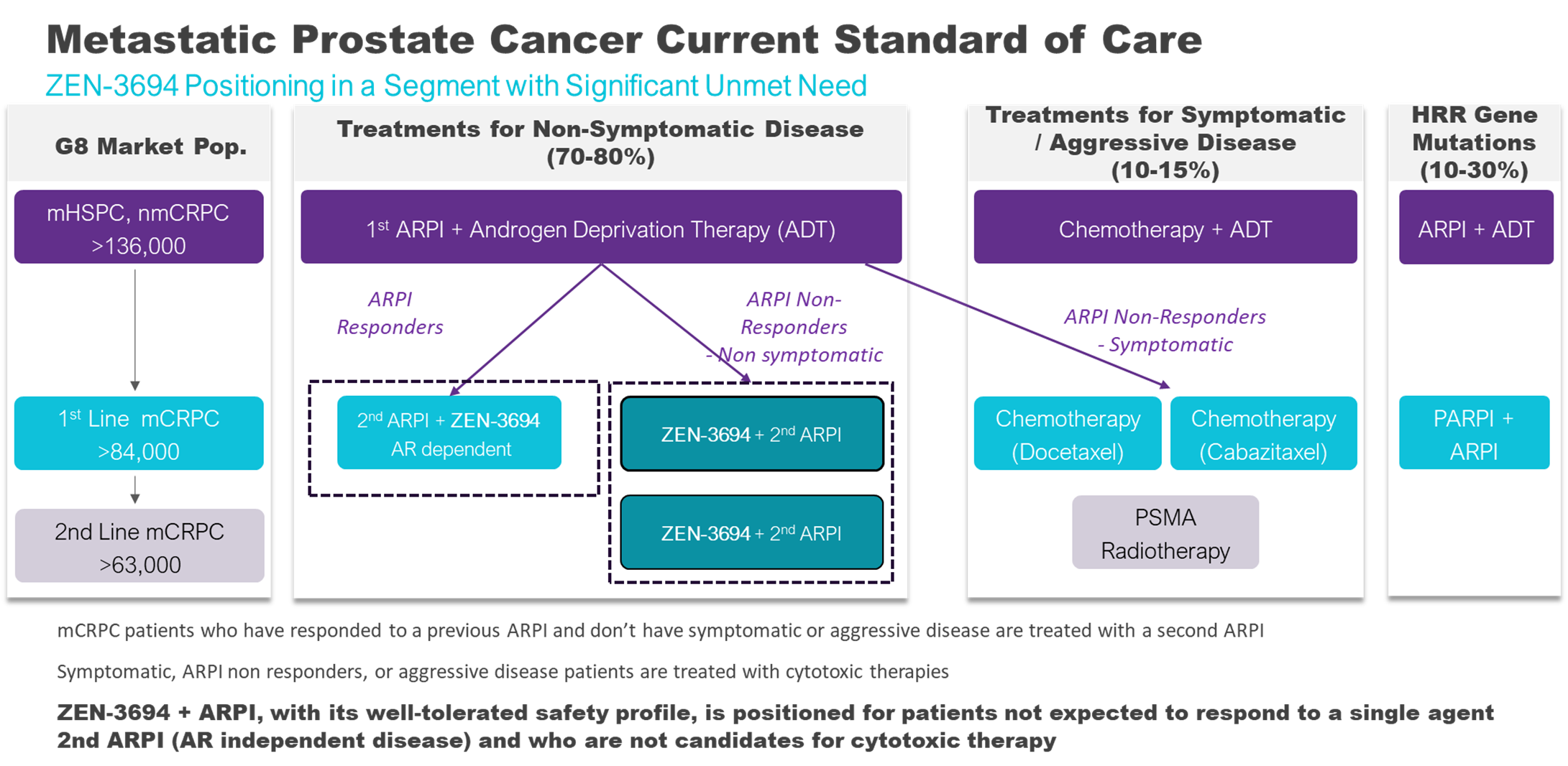
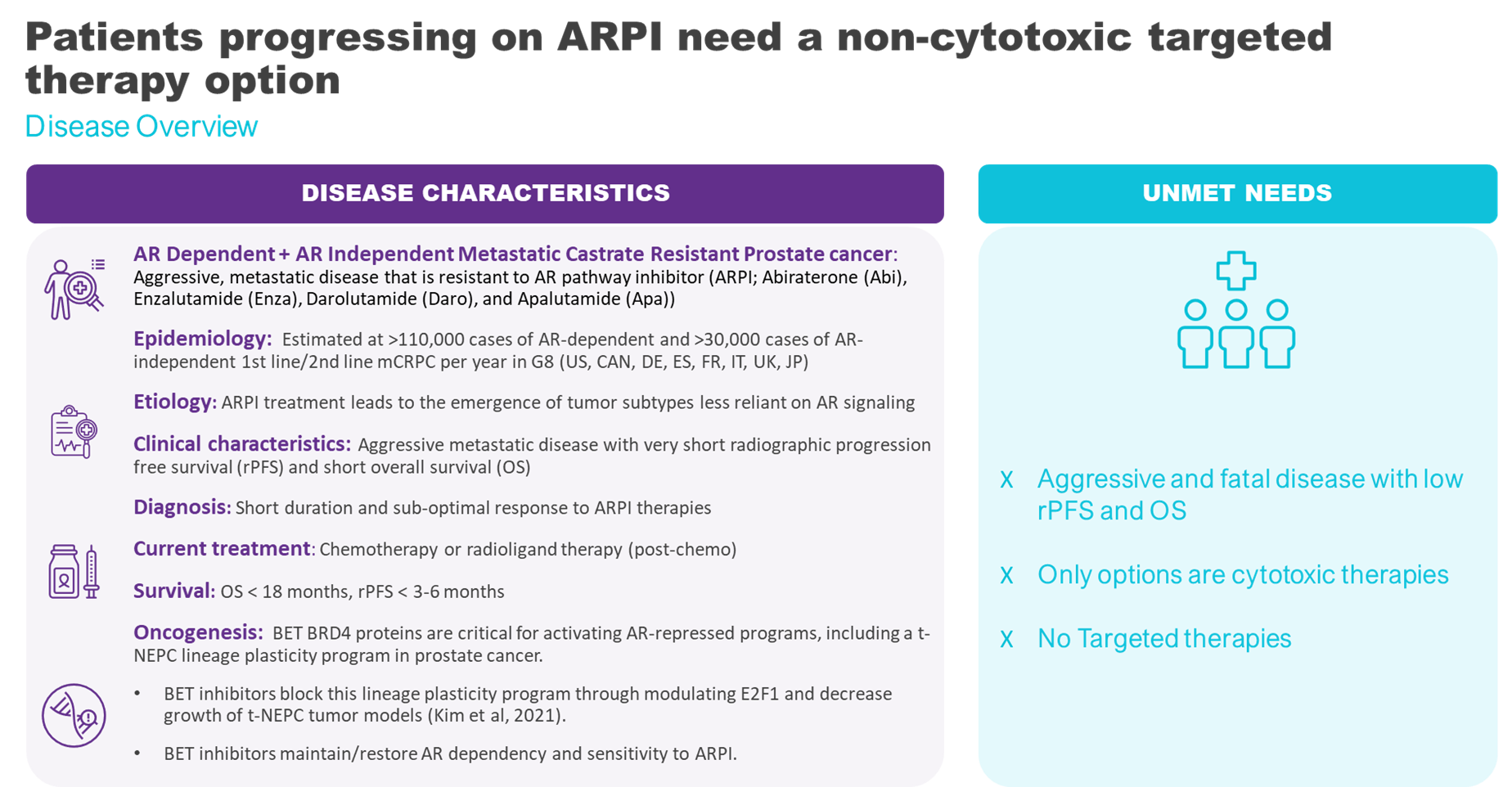
Prostate cancer is the second-most frequently diagnosed cancer and cause of cancer deaths in men. A considerable proportion of patients with advanced disease will eventually progress to mCRPC. The incidence is rising due to the improved survival of men with earlier stages of prostate cancer, leading to a larger pool of individuals at risk of developing castration resistance. The estimated incidence is greater than 136,000 cases of metastatic castration resistant prostate cancer per year in G8 countries (US, CAN, DE, ES, FR, IT, UK, JP).
mCRPC Diagnosis
mCRPC is diagnosed by a prostate-specific antigen (PSA) monitoring. A rising PSA level despite castration therapy is a primary indicator that prostate cancer has become castrate resistant. Imaging tests such as bone scans, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) scans are used to detect metastases. Histological analysis of a biopsy sample from the metastatic site may be used to confirm the presence of cancer.
mCRPC Prognosis
The prognosis for patients with mCRPC has historically been poor, with a median overall survival of approximately two years. Patients diagnosed with metastatic disease at the initial prostate cancer diagnosis tend to have a worse prognosis after developing CRPC compared to those who were initially non-metastatic.
mCRPC Treatment
The therapeutic landscape for mCRPC is rapidly evolving. The treatment for metastatic castration resistant prostate cancer had been continued androgen deprivation therapy and second-generation ARPI such as abiraterone acetate, and androgen receptor antagonists such as enzalutamide, and apalutamide. Chemotherapy, radiotherapy, radioligand therapy, and PARP inhibitors for mCRPC patients with specific gene mutations may also be used. As ARPI are now used in earlier settings of prostate cancer, tumors are generally resistant to ARPI as they enter the mCRPC stage. New therapies such as BETi are needed at this stage to address resistance to ARPIs.
BET Inhibitors and mCRPC
We conducted a first-in-human Phase 1b/2 study of ZEN-3694 in combination with enzalutamide in patients with metastatic castration resistant prostate cancer and prior progression on one or more ARPI (second line mCRPC). Results demonstrated that ZEN-3694 is well tolerated and has encouraging preliminary efficacy in combination with enzalutamide in patients with this aggressive and difficult to treat form of prostate cancer. The median radiographic progression-free survival (rPFS) in the overall study was 10 months which is promising when comparing to less than 6 months for single agent ARPI.
The efficacy was particularly impressive in the subset of patients that had previously responded poorly to abiraterone and whose cancer was more likely to progress independently of AR signalling. These AR-independent patients are expected to have a poor response to enzalutamide – a current standard of care therapy, because the tumor is insensitive to inhibiting AR. Clinical and pharmacodynamic data suggest that the combination of ZEN-3694 and enzalutamide may be able to thwart resistance mechanisms and re-sensitize patients to ARPI. An ongoing expanded Phase 2b randomized trial evaluating ZEN-3694 in combination with enzalutamide (compared with enzalutamide alone) in randomized patients that have previously responded poorly to abiraterone is ongoing in collaboration with Astellas and Newsoara Biopharma and the interim results are promising. Additionally, the combination is also showing promise in patients that have responded to abiraterone and their tumors are still considered androgen signalling dependent. The data suggests that the combination of ZEN-3694 and enzalutamide has an effect on a broader population, those that have responded to abiraterone and those that did not respond to abiraterone.
Preclinical evidence suggests that combining BET inhibitors with checkpoint inhibitors in prostate cancer may improve outcomes compared to either agent alone (synergy). An ongoing Merck sponsored investigator initiated trial led by Dr. Rahul Aggarwal at UCSF evaluating ZEN-3694 + enzalutamide and checkpoint inhibitor pembrolizumab has shown promising results.