ZEN-3694 development has not been limited by off target toxicities that have hampered the development of other BETi. ZEN-3694 can be taken orally once daily with a manageable safety profile, allowing for chronic dosing. Over 550 patients have been dosed with ZEN-3694. ZEN-3694 maintains target engagement for 12 out of 24 hours allowing for an optimal balance between efficacy and safety supported by pre-clinical in vivo data. The PK/PD characterization for Zenith’s mCRPC clinical studies is robust compared with other clinical BETi programs. This has helped Zenith select a recommended Phase 2 dose (RP2D) based on target modulation, safety, and activity. ZEN-3694 has shown equivalent or better target modulation in patients compared with other BETi with a superior safety profile.
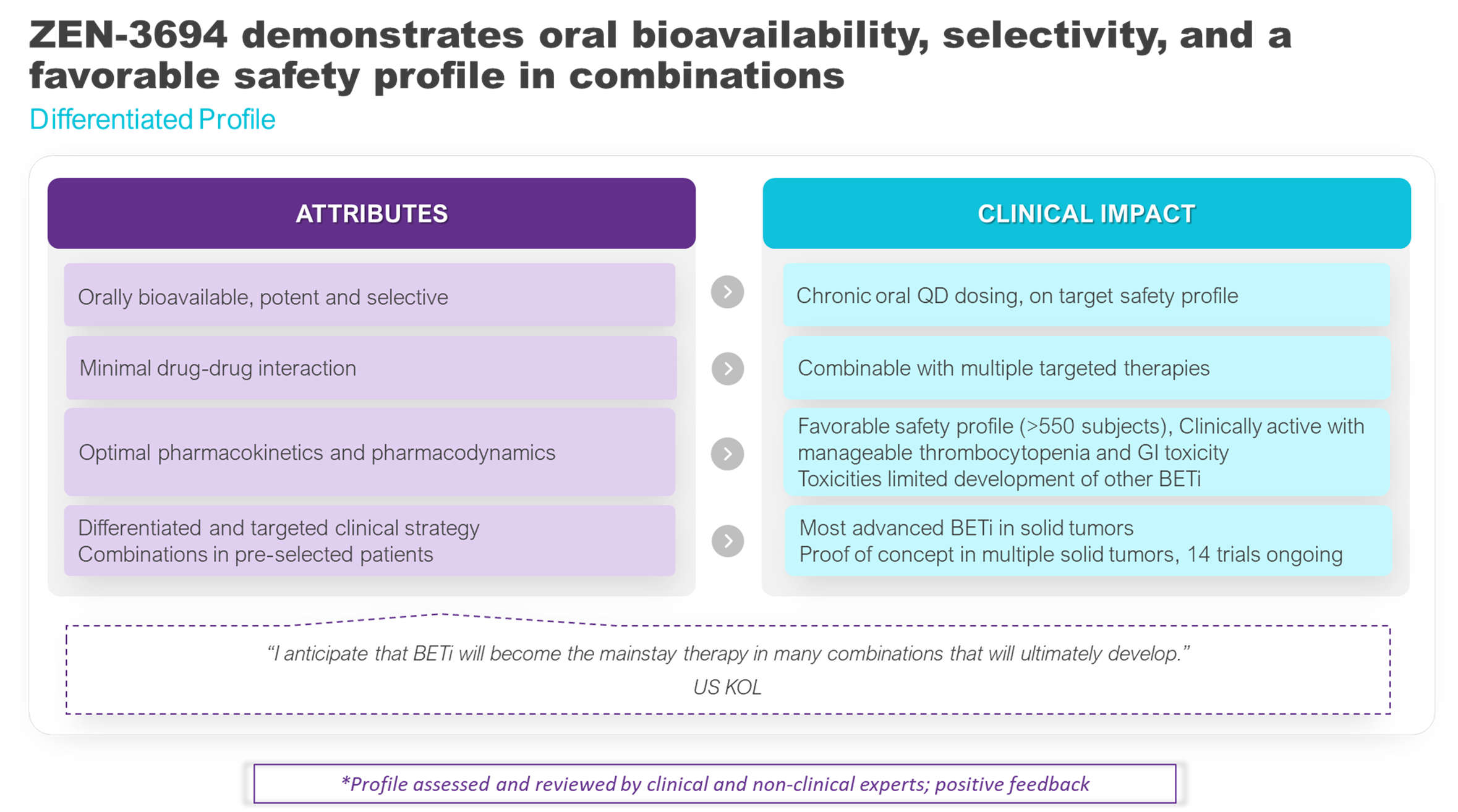
ZEN-3694 has minimal drug-drug interaction potential making it combinable with multiple targeted therapies including CKD4/6 inhibitors, androgen receptor pathway inhibitors, checkpoint inhibitors, PARP inhibitors, MEK inhibitors, HDAC inhibitors, and chemotherapy. Zenith has also implemented a differentiated clinical strategy combining with targeted therapies in various oncology indications to overcome resistance and improve efficacy and durability of response. A key attribute of Zenith’s clinical strategy is selecting subgroups of patients that are most likely to respond to treatment. With the differentiated profile of ZEN-3694 and Zenith’s patient enrichment clinical strategy, ZEN-3694 is now the most advanced BET inhibitor in solid tumors.
